Monotropoid mycorrhizae, in common with arbutoid mycorrhizas, share features typical of ectomycorrhizas and ectendomycorhizas. Monotropoid mycorrhizas have a mantle that is sometimes very thick, and a Hartig net confined to the epidermis (para epidermal).
Although the plant species that form this category of mycorrhiza also belong to the large order Ericales, structural features of the mycorrhiza separate it from ericoid and arbutoid mycorrhizas (Duddridge and Read 1982). They also possess a unique feature, the invasion of epidermal cells by short hyphae originating from the Hartig net or inner mantle.
These structures referred to as fungal pegs (Figures 1,2), form along the outer tangential wall of epidermal cells in Monotropa species but at the base of the radial wall of epidermal cell Pterospora and Sarcodes sanguinea (Robertson and Robertson 1982). Host cells respond by depositing additional cell wall material, in finger-like projections, around each peg.
The second feature of monotropoid mycorrhizas is that plants forming this type of mycorrhiza are all achlorophyllous, heterotrophic (non-photosynthetic) species; they depend on symbiotic fungal associations that act as linkages to neighboring autotrophic (photosynthetic) trees or shrubs for their carbon acquisition.
In an extensive review of heterotrophic plants and their fungal associations, Leake (1994) suggests the term myco-heterotrophic best describes these associations. Others refer to these plants as epi-parasites, because they indirectly “parasitize” surrounding trees. It is generally accepted that the term saprophyte should be avoided.
Plant species involved in Monotropoid Mycorrhizae
Depending on the taxonomic system used, all genera are found either in the family Monotropaceae or in the clade Monotropoideae, in the family Ericaceae. Regardless, ten genera (Allotropa, Cheilotheca, Hemitomes, Monotropa, Monotropantham, Monotropsis, Pityopus, Pleuricospora, Pterospora, Sarcodes) are presently recognized (Leake 1994), all but three of these genera being mono typic (i.e., with only one species).
The Asian genus Cheilotheca has four recognized species, and the genera Monotropa and Pleuricospora each have two species. The distribution of the group is primarily north temperate with the largest number of species in western North America. Most of the research, in terms of mycorrhizal associations, has been with the genera Monot-ropa, Sarcodes, and Pterospora.
Fungal species involved in Monotropoid Mycorrhizae
Over the years, a number of studies have documented the fungi possibly associating with members of the Monotropoideae; with the advancement of molecular approaches, the identities of some fungi have been confirmed (Bidartondo and Bruns 2001).
The plant genera examined are associated with members of five families of basidiomycetes, fungi also known to form ectomycorrhizas with numerous tree species. The interesting trend is that each plant genus or species, where there are more than one species in the genus, has become highly specialized in terms of its fungal associates.

For example, Monotropa hypopitys appears to form monotropoid mycorrhizas only with species in the fungal genus Tricholoma, whereas Monotropa uniflora forms mycorrhizas with Russula species and other members in the family Russulaceae (Bidartondo and Bruns 2001; Young et al. 2002).
This specificity is true regardless of the geographical distribution of the plant species. The monotypic plant species, Sarcodes sanguine, and Pterospora andromedea, shown to be closely related phylogenetically, form mycorrhizas with fungal species in the genus Rhizopogon, with each plant species showing a preference for particular Rhizopogon species.
Bruns and Read (2000) isolated Rhizopogon species from mature plants of both S. sanguinea and P. andromedea and used these, as well as a number of other ectomycorrhizal fungal species, in germination trials with both plant species.
Of the fungi tested, only Rhizopogon isolates were effective in promoting seed germination; isolates originating from either plant species were effective in stimulating germination in both plant species. Seeds without a Rhizopogon isolate did not germinate.
This study shows the importance of the presence of the appropriate fungus for seed germination in these myco-heterotrophic species.
Development and structure
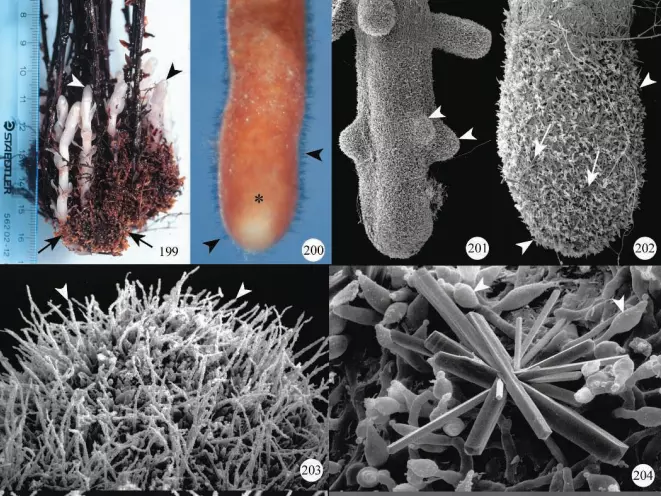
The most detailed observations of the development and structure of monotropoid mycorrhizas have been obtained from studies on the genus Monotropa. In M. uniflora, and M.hypopitys, clusters of roots form, each root becoming ensheathed with fungal hyphae resulting in a well-developed mantle.
Details of the mantle can best be seen using scanning electron microscopy. Frequently, large calcium oxalate crystals are deposited among the outer mantle hyphae (Figure 204) and along the surface of hyphae within the mantle.
Cystidia may be present, depending on the fungal species. Rhizo-morphs are sometimes present, and have been traced in vivo from the surface of M. hypopitys mycorrhizas to the roots of neighboring pine trees (Duddridge and Read 1982).
The mantle is usually multi-layered with a compact inner mantle (Figure 210). A para epidermal Hartig net develops and hyphae originating from this or from the inner mantle hyphae penetrate into epidermal cells to form fungal pegs.
Figure 210. Light microscopy of a paranormal section of Monotropa uniflora mycorrhiza showing the compact inner mantle (M), and sectional views of hyphal pegs (arrowheads) in epidermal cells.
Transmission electron micrograph showing details of a hyphal peg. Finger-like depositions of host cell wall material (arrowheads) are evident.
These fungal pegs, one per epidermal cell, are encased by wall material synthesized by the host plant. The wall is laid down unevenly so that each peg has wall projections enveloped by the host plasma membrane (Figure 211), a structure similar to that in transfer cells described in many plant tissues.
The formation of plasma membrane around. these wall ingrowths increase the surface area of this membrane; the increased surface area may be important in nutrient exchange at this interface.
Mitochondria and profiles of the endoplasmic reticulum, often suggestive of increased metabolic activity, are always found in the vicinity of these walls in growths. The pegs form along the outer tangential wall of epidermal cells in Monotropa. In any cross-section of a root, most epidermal cells show the development of these structures.
With time, each peg undergoes changes in that the host wall surrounding the tip breaks down; the fungal peg eventually degrades and, concomitantly, changes occur in the surrounding plasma membrane to form a sac-like structure.
The contents of the fungal peg apparently move into this membranous structure. In one study of M. hypopitys, certain stages in the development of mycorrhizas were documented to correlate with stages in plant development (Duddridge and Read 1982).
Mantle and Hartig net formation and the first stages of peg formation in epidermal cells occurred before the shoot emerged above ground. At this time, glycogen was stored in the Hartig net and mantle hyphae.
As shoots emerged, the number of fungal pegs increased dramatically, and, with time, the number of pegs with degraded tips dominated the population. Following the maturation of flowers and during seed set, degeneration of the fungal pegs, Hartig net, and mantle hyphae occurred. Monotropoid Mycorrhizae
Roots of Pterospora andromedea also occur in coralloid clusters (Figure 212) with each root forming several laterals (Figure 213). The mantle covers the main root and lateral roots (Figure 214); hyphae of the external mantle are very irregular in shape and small crystals are present on their surface (Figures 215, 216).
The mantle is multi-layered and the Hartig net is para epidermal. Hyphae from the Hartig net penetrate epidermal cells, usually towards the base of radial walls, to form fungal pegs.
General descriptions of field-collected mycorrhizas of Sarcodes sanguinea were included as part of a study on the transport of radioactive compounds from autotrophic trees to this species (Vreeland et al. 1981). S.sanguinea mycorrhizal roots developed in clusters or coralloid masses, within which each mycorrhizal root tip formed a mantle and para epidermal Hartig net.

In a more detailed ultrastructural study of this species, as well as Pterospora and romedea, Robertson (1982) illustrated the coralloid clusters of roots for both species and provided details of the interface between fungus and root cells.
Functions
All plant species having monotropoid mycorrhizas are non-photosynthetic and myco-heterotrophic. Increasing evidence suggests that these specialized plants have evolved a mechanism to obtain photosynthates by forming mycorrhizas with fungi that are also able to associate with neighboring photosynthetic trees or shrubs.
Using radioisotopes, it has been demonstrated that photosynthates (Björkman 1960) can move from trees to Monotropa hypopitys plants and that phosphorus (Vreeland et al. 1981) can move from trees to Sarcodes sanguinea.
The mechanism of transport of nutrients from the mycorrhizal fungus into the roots of monotropoid species remains uncertain.
The elaborate wall ingrowths and the concomitant development of the plasma membrane around the fungal pegs are circumstantial evidence that this region may be the site of the transfer of compounds from the fungus to the epidermal cells. The role of the Hartig net in transferring metabolites has not been explored.

Does anybody know whether I am able to purchase JUSTKRATOM White Maeng DA Kratom Powder (justkratomstore.com) at TKO Vapor, 654 Mill Creek Mall #9021-T, Erie, PA, 16565?