Orchid mycorrhiza is unique because they occur only within the family Orchidaceae, one of the largest families of flowering plants. Although they share some characteristics with other mycorrhizal types (e.g., root cells are colonized by fungi), they differ in that fungal associations are essential for both seed germination and seedling establishment in nature (Rasmussen 1995; Peterson et al. 1998).
The main defining characteristic of orchid mycorrhizas is the formation of complex hyphal coils (pelotons) within host plant cells. Orchid mycorrhizas can, therefore, be considered within the broad category of endomycorrhizas.
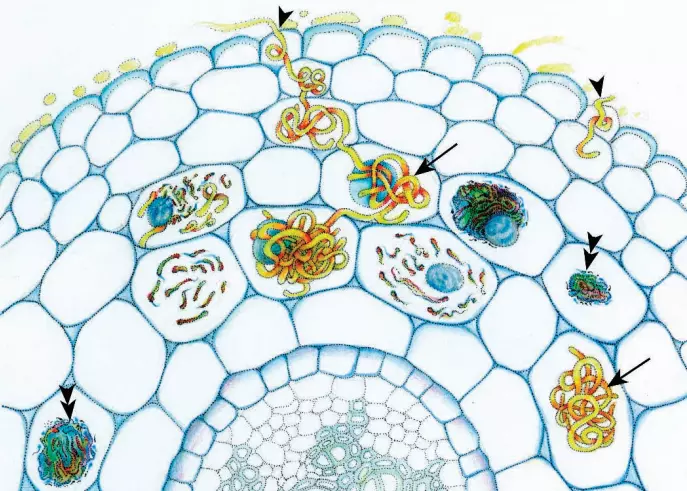
Although it is often presumed that orchid mycorrhizas are mutualistic symbioses, there is no evidence, to date, that the fungus benefits from the association. The suggestion has been made (Smith and Read 1997) that orchids may have evolved a unique relationship with their associated fungi that involves parasitism, in this case by the host plant on the fungus.
Plant species involved
The family Orchidaceae, consisting of approximately 450 genera and over 17,000 species, has a worldwide distribution, but with the largest number of species in the tropics. Various life forms occur: terrestrial, epiphytic, and lithophytic (plants growing on cliffs or rock faces), with a few species of Rhizanthella living entirely below ground until flowering (Dixon 2003).
Several orchid Mycorrhizal species remain achlorophyllous during their entire life cycle and are, therefore, myco-heterotrophic, depending on fungi for carbon compounds derived either from the breakdown of organic matter in the soil or from mycorrhizal linkages with autotrophic plants.
The diversity in floral forms and the specialized pollination mechanisms have
attracted considerable attention to the Orchidaceae by scientists, amateur orchid growers, and horticulturalists. Many species are grown commercially for the floriculture industry, and the genus Vanilla is grown for its extract from seed pods which is used as a flavouring in the food industry.
Many orchid Mycorrhizal species can now be propagated from seeds in vitro by supplying the germinating seeds with a source of simple sugars or providing the appropriate fungus using a medium containing a complex organic source.
This is of particular importance since species or hybrids with showy and unusual flowers can be produced in large numbers for the flower market. Also, attempts are being made to reintroduce into their native habitats seedlings of endangered species that have been propagated in vitro.
A major difficulty with this method is the loss of large numbers of seedlings as they are removed from culture flasks and transplanted into potting soil. Trials are being conducted to inoculate seedlings with appropriate mycorrhizal fungi to overcome this problem at this step in the production process.
Fungal species involved
Fungi can be isolated rather easily from the roots of orchids and most isolates grow well on various culture media. One method involves removing individual pelotons from colonized root cortical cells and then plating these on sterile media. Hyphae growing out from these pelotons are then subcultured.
This method avoids most contamination that could come from other fungi that may be present on the surface or within root tissues. It has been more difficult to isolate fungi from germinating seeds and protocorms. However, it is now possible to place mesh bags containing orchid seeds in soil to act as baits for mycorrhizal fungi (see Rasmussen 1995).
Most of the early descriptions of fungal isolates were based on morphological characteristics of the fungal colonies grown on agar medium in combination with structural characteristics of the hyphae (Currah et al. 1997, Figures 230, 231).
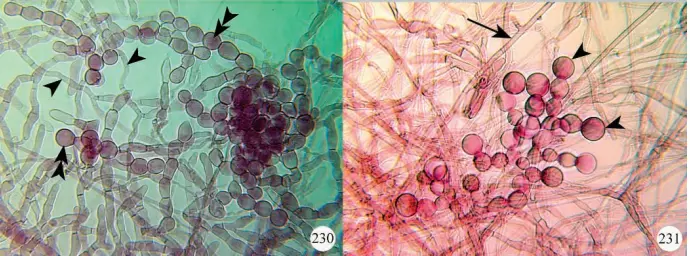
inquilina isolated from a root of Paphiopedilum charlesworthii. Figure 231. Runner hypha (arrow) and monilioid cells (arrowheads) of Epulorhiza repens isolated from a root of Paphiopedilum hirsutissimum.
Based on this, fungi isolated from the roots of orchid species were grouped in the form of the genus Rhizoctonia. Many of these were capable of stimulating seed germination of a number of orchid species (Ras-mussen 1995).orchid Mycorrhizae
Following intensive culturing of isolates, sexual stages (teleomorphs) have been produced for several isolates and it is now known that there are several anamorphs (asexual stages) with associated teleomorphs that are symbionts with orchids (Currah et al. 1997; Rasmussen 1995).
The identification of fungal symbionts in orchid mycorrhizas has also been enhanced recently by the use of molecular methods.
Some anamorphs that are known to be pathogens on a variety of plant hosts are able to form symbiotic associations with orchids, at least in sterile culture experiments. Although the systematics of the fungi associated with orchids is still in progress, some facts are known.
For example, autotrophic orchid species (those that have chlorophyllous seedlings and adults) associate with different fungal species than myco-heterotrophic species (those that remain achlorophyllous throughout their life cycle).
Autotrophic terrestrial orchids form mycorrhizas with basidiomycete anamorph genera such as Ceratorhiza, Epulorhiza, and Moniliopsis (corresponding teleomorph genera are Ceratobasidium, Tulasnella, and Sebastian, and Thanatephorus, respectively) (Currah et al. 1997).
Many of these orchid fungal symbionts produce enzymes such as cellulases and polyphenol oxidases that enable them to break down soil organic matter into simple sugars that may be utilized by both partners in the symbiosis.
If necessary these fungi could potentially survive as saprophytes in the soil and act as viable sources of inoculum for developing orchids.
Myco-heterotrophic orchid genera such as Corallorhiza, however, are dependent on fungal symbionts for their nutrition and form mycorrhizal associations with several basidiomycete fungal genera known to form ectomycorrhizas with tree species. Among these, species of Russula, Thelephora, and Tomentella are important.
As described for the plant members of the Monotropoideae, myco-heterotrophic orchid genera are able to form mycorrhizas with fungal species that link them with adjacent trees or shrubs and use these hyphal linkages between their roots and tree roots to gain photosynthates produced by the autotrophic host.
Fungal linkages between orchids and tree and (or) shrub species are being reported more frequently and are indicative of the complex ecological relationships that exist among plants and other organisms in nature.
Orchid seed germination and protocorm formation
1. Seed and protocorm structure
All orchid species form numerous minute seeds per capsule and, because of their small size, are referred to as ‘dust seeds’ (Figure 232). Seed coats display various patterns (Figure 233), some of which may be related to their dispersal by wind.
Each seed contains a minute undifferentiated embryo lacking a root and shoot apical meristem (Figure 234).In addition, the embryo cells store lipids and proteins (Figure 235) since endosperm is not present.
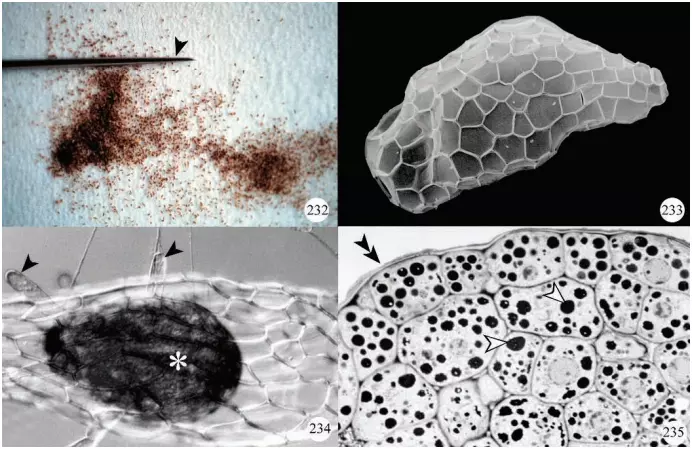
needle. Photo courtesy of Dr. Yukari Kuga-Uetake. Figure 233. Scanning electron micrograph of a seed of Listera australis showing the pattern of testa cells. Figure 234. Seed of Platanthera hyperborea showing the undifferentiated embryo (*) and epidermal hairs (arrowheads). Figure 235. Transverse section through the embryo of a Platanthera hyperborea seed showing deposits of protein (arrowheads). The outline of the seed coat (testa) is indicated by double arrowheads.
These unique structural characteristics require that seeds must first become colonized by an appropriate fungal species that provides necessary carbohydrates for further development of the embryo into a structure called a protocorm (see Figures 236–239 for the sequence of events that occur from seed to protocorm to seedling).
The protocorm is composed of parenchyma cells, some of which eventually initiate a shoot apical meristem. The first adventitious root primordium is initiated after the first leaf primordium forms from the shoot apical meristem.
An unusual feature of orchid seedlings is that a primary root system is lacking so that all roots formed are adventitious in origin. Since the protocorm and the seedling that develops from it often remain below ground for more than one growing season, this phase of the life cycle is myco-heterotrophic.
2. Early colonization events in orchid Mycorrhizae
Most of the information concerning the colonization of orchid embryos comes from in vitro experiments, primarily because of the difficulty in following the early developmental stages in soil.
Recently, the use of small mesh bags containing seeds, and placing these in the soil so that seeds can become colonized by fungal hyphae, has led to some information on natural infection processes (see Rasmussen 1995).
A hypha of a compatible fungal species contacts an imbibed seed and enters either through the suspensor end of the embryo or through epidermal hairs (some authors refer to these as rhizoids) that develop from the embryo after imbibition and the splitting of the seed coat (Figure 234).
Once inside the embryo, the fungus forms hyphal coils (pelotons) in the embryo cells. Most cells, except those from which the shoot apical meristem develops (and in some species, the epidermis), become colonized.
The hyphal coil is separated from the host cell cytoplasm by a perifungal membrane which is a modification of the host plasma membrane.
Entry of fungal hyphae into embryo and protocorm cells triggers nuclear enlargement within host cells this event is accompanied by several cycles of DNA synthesis and changes in the host cell cytoskeleton.
Fungal hyphae pass from cell to cell presumably by the production of hydrolytic enzymes that break down localized areas of the cell wall. Hyphae that pass through the host cell wall have a very narrow diameter compared to peloton hyphae.
The embryo increases in size through cell divisions and cell enlargement to produce the protocorm. As this happens, the first formed pelotons undergo degradation to form clumps of lysed hyphae within cells. It is now known that these degraded hyphae are isolated from the host cell cytoplasm by wall constituents synthesized by the host cell (Peterson et al. 1996 ).
This may be important in preventing host cytoplasm damage during this lytic process and may allow the cell to become invaded more than once by fungal hyphae.
Seedling establishment and mature plants (Orchid Mycorrhizae)
Once the shoot apical meristem is established, leaves form, and root primordia are initiated. Many orchid species remain below ground for more than one season or, in some cases, throughout the vegetative phase of their life cycle.
Consequently, they depend on their fungal partner to supply carbohydrates for growth. Eventually, in chlorophyllous orchids, leaves emerge above ground and these are able to synthesize their own sugars.
As roots elongate into the soil they are colonized by symbiotic fungi, and cortical cells develop pelotons. It appears that the roots are colonized de novo from fungal propagules in the soil, and not by the fungus passing from the protocorm.
Thus different fungal species may colonize the root and the protocorm, and more than one fungal species may invade the same root. There are few studies of the colonization of orchid root cells by mycorrhizal fungal hyphae, but reports indicate that fungi may enter directly through epidermal cells or root hairs.
In orchid roots with an exodermis consisting of short and long cells, hyphae enter through the short cells to gain access to the remainder of the cortex.
This is analogous to what occurs in roots with exodermis that are colonized by AM fungi. Aerial roots of epiphytic orchids have a multi-layered epidermis (the velamen) that can become colonized by a variety of organisms (Zelmer 2001).
These roots also have an exodermis consisting of short and long cells and, as above, mycorrhizal fungal hyphae access the cortex by passing through the short cells. Pelotons occurring in root cells are degraded and isolated from the host cytoplasm, following which a cortical cell can be reinvaded by the fungus.
Terrestrial orchid species may develop a variety of underground organs including roots, root tubers (roots specialized for storage of starch), and rhizomes. There are reports of all of these structures being associated with mycorrhizal fungi, but the highest levels of colonization are generally found in the roots.
Field-collected roots that appear white are usually filled with starch and colonization levels of mycorrhizal fungi are often low. Older roots that appear dark in color due to the accumulation of phenolics in epidermal cells are usually heavily colonized by mycorrhizal fungi.
Functioning of orchid mycorrhizas
1. Seed germination and protocorm establishment
Experiments using several orchid species have shown that protocorm growth occurs following the formation of the first pelotons. Radioactive tracer studies indicate that the transfer of carbon compounds and phosphorus into growing protocorms occurs via fungal hyphae.
Trehalose, a fungal sugar, is translocated to protocorms where it is metabolized into other carbohydrates, including sucrose. Stimulation of protocorm growth during fungal infection appears primarily due to the translocated sugars (see Smith and Read 1997).
After exposing fungal mycelium connected to protocorms to a source of radioactive phosphorus, radioactivity was found in protocorm tissues.
Although some circumstantial evidence exists regarding the importance of mycorrhizal fungi in nitrogen acquisition by orchids, there is no direct evidence that fungal hyphae transport nitrogen compounds from the soil to the developing protocorms.
It is known that the form and concentration of nitrogen provided to germinating seeds and protocorms in vitro can have a profound effect on the symbiotic relationship. In some instances, particularly at high levels of nitrogen, the fungus can proliferate and parasitize protocorms resulting in protocorm death (Beyrle et al. 1995).
The site at which sugars and mineral nutrients are transferred from the fungus to the orchid cells is not known. It is likely, however, that since a perifungal membrane surrounds peloton hyphae, the transfer of nutrients across this membrane occurs before fungal cell death and subsequent digestion by host cells.
Alternately, nutrients accumulated within fungal hyphae could be released into the host cell upon hyphal lysis; the plant could then utilize these.
2. Seedlings and mature plants
Although numerous reports document the occurrence of typical orchid mycorrhizal structures in the roots of terrestrial and epiphytic orchids (Rasmussen 1995; Smith and Read 1997), few have considered seedling and mature chlorophyllous plants in terms of the functioning of the mycorrhizal fungi.
In fact, there has been some debate as to whether these orchids require mycorrhizal fungi. The morphology of the root systems of chlorophyllous terrestrial orchids, however, is suggestive of the dependency of these plants on fungi for nutrient acquisition.
Roots are generally fleshy, unbranched, and few in number, all characteristics of plants with a high dependency on mycorrhizal fungi (Baylis 1975).orchid Mycorrhizae
In addition to questions pertaining to the functional aspects of these mycorrhizas, aspects related to the extent of the extraradical mycelium and its interaction with biotic and abiotic components of the soil need to
be explored. Experimental work with radioactive tracers has shown that Goodyera repens seedlings were more dependent than older plants on sugars supplied by the mycorrhizal fungus (Alexander and Hadley 1985).
These authors also showed that sugars were not transferred from the plant to the fungus. In the same orchid species, 32P was transported via fungal mycelium to mature plants, and at low concentrations of P, there was enhanced growth of these plants compared to plants whose associated fungal mycelium had been interrupted by a fungicide treatment (Alexander et al. 1984).
References
- https://en.wikipedia.org/wiki/Orchid_mycorrhiza
- Mycorrhizal Symbiosis: Sally E. Smith, David J. Read – 2010
- Mycorrhiza: Role and Applications
