Glycolysis is a vital metabolic pathway present in nearly all living organisms that involves the breakdown of glucose to generate energy as ATP.
This ancient evolutionarily conserved process forms the foundation for both aerobic and anaerobic respiration and serves as a key stage linking carbohydrate metabolism to fat and protein metabolism.

Understanding the intricacies of glycolysis provides crucial insight into core biochemical pathways underlying cellular bioenergetics.
This guide will explore the 10-step process of glycolysis, outlining the enzymes and intermediates involved, regulation, energetics, and connection to downstream metabolic fates.
Overview of Glycolysis
Glycolysis is the metabolic breakdown of glucose into two molecules of pyruvate via a series of intermediate compounds. It occurs in the cytosol of cells and does not directly require oxygen. We can summarize the overall reaction as:
Glucose + 2 NAD+ + 2 ADP + 2 Pi → 2 Pyruvate + 2 NADH + 2 H+ + 2 ATP + 2 H2O
While glycolysis yields only two ATP molecules per glucose, the two NADH generated carry electrons that drive oxidative phosphorylation to produce far more ATP when oxygen is available. Alternatively, NADH powers fermentation pathways.
They regulate glycolysis based on cellular energy demands and redirect intermediates into other biosynthetic pathways. As the first stage of cellular respiration, glycolysis primes glucose for further catabolism and provide precursors for anabolism.
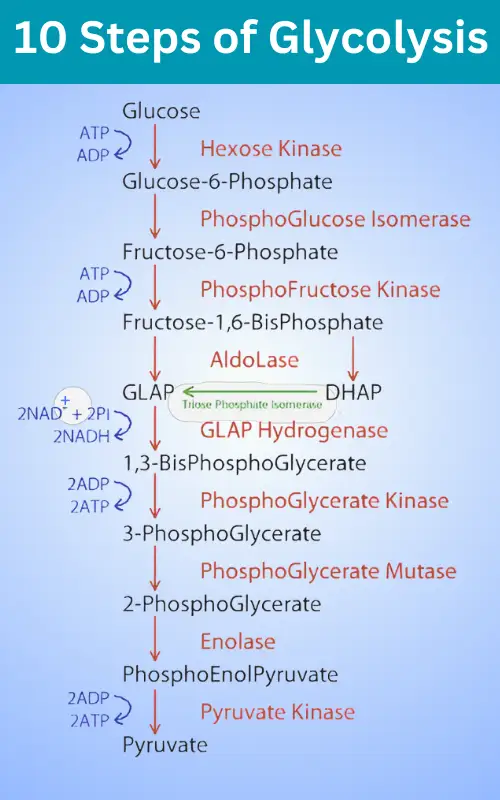
The 10 Steps of Glycolysis
Glycolysis involves two distinct phases – the energy investment phase consumes ATP, and the energy generation phase produces ATP and NADH.
Energy Investment Phase
The first half of glycolysis requires energy input to convert glucose into more reactive phosphorylated intermediates.
Step 1:
Hexokinase transfers a phosphate from ATP to glucose, forming glucose-6-phosphate. This traps glucose inside the cell and destabilizes bonds to start the breakdown.
Step 2:
Phosphoglucose isomerase rearranges glucose-6-phosphate into fructose-6-phosphate..
Step 3:
A second ATP phosphorylate fructose-6-phosphate into fructose-1,6-bis phosphate via phosphofructokinase. This commits the intermediate into glycolysis.
Step 4:
The enzyme aldolase cleaves fructose-1,6-bis phosphate into dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP).
Step 5:
Triose phosphate isomerase inter converts DHAP into GAP.
Energy Generation Phase
The second half of glycolysis extracts ATP and high-energy electrons from phosphorylation of intermediates.
Step 6:
Glyceraldehyde-3-phosphate dehydrogenase catalyzes the oxidation of GAP to produce 1,3-bis phosphoglycerate and reduce NAD+ to NADH.
Step 7:
Phosphoglycerate kinase generates ATP by phosphorylating ADP, yielding 3-phosphoglycerate.
Step 8:
Phosphoglycerate mutase moves the phosphate group, forming 2-phosphoglycerate.
Step 9:
Enolase dehydrates 2-phosphoglycerate into phosphoenolpyruvate (PEP).
Step 10:
Pyruvate kinase transfers PEP’s phosphate to ADP, forming a second ATP and pyruvate.
Energetics and Regulation of Glycolysis
- Every glucose molecule starts glycolysis by expending 2 ATP but yields 4 ATP, resulting in a net gain of 2 ATP per glucose.
- It also produced two NADH molecules, carrying high-energy electrons.
- Regulatory mechanisms control flux by regulating the irreversible steps – hexokinase, phosphofructokinase, and pyruvate kinase.
- ATP inhibits phosphofructokinase when energy status is high to prevent waste of glucose.
- Fructose-2,6-bis phosphate overrides ATP inhibition of phosphofructokinase to stimulate glycolysis.
- Pyruvate and citrate show sufficient downstream capacity to process pyruvate, influencing the glycolytic rate.
Connection to Other Metabolic Pathways
Glycolysis interconnects with many other pathways through shared intermediates:
- Glucose-6-phosphate can enter the pentose phosphate pathway for NADPH and nucleotide synthesis.
- DHAP and GAP provide precursors for gluconeogenesis and the ** Calvin cycle**.
- 1,3-Bis phosphoglycerate has a non-glycolytic role in red blood cells.
- 3-Phosphoglycerate is involved in serine synthesis.
- PEP takes part in the shikimate pathway generating aromatic amino acids.
- Pyruvate can enter the TCA cycle or contribute to amino acid synthesis.
Metabolic Fates of Pyruvate and NADH
The ultimate fate of glycolytic end products depends on cellular oxygen availability:
- In aerobic cells, pyruvate and NADH enter mitochondria to fuel the TCA cycle and electron transport chain generating far more ATP.
- In anaerobic cells lacking mitochondria, pyruvate undergoes fermentation to recycle NAD+ and continue glycolysis. These are present in muscles during intense activity.
- Common fermentation pathways include lactic acid fermentation in animals and alcoholic fermentation in yeast/plants.
Dysregulation in Disease
Glycolysis is regulated tightly to meet metabolic demands. Defects in glycolytic enzymes or dysregulation manifest in certain diseases:
- Diabetes – Insufficient insulin leads to impaired glucose uptake and utilization by cells.
- Cancer – Malignant cells exhibit amplified glycolysis rates despite oxygen availability (Warburg effect).
- Genetic disorders – Mutations in glycolytic enzyme-encoding genes underlie various metabolic diseases.
- Toxic accumulation of intermediates – Buildup of upstream metabolites inhibits glycolysis.
Thus, proper glycolytic function is significant for maintaining energy homeostasis and health.
FAQs About Glycolysis
Q: What is the net gain of ATP from one glucose molecule?
A: Four ATP are produced but two are invested, resulting in a net gain of two ATP per glucose through substrate-level phosphorylation in glycolysis.
Q: Where in the cell does glycolysis occur?
A: Glycolysis takes place in the cytosol of both prokaryotic and eukaryotic cells rather than within any organelle.
Q: What starts glycolysis in cells?
A: The presence of glucose and a mechanism for glucose uptake and phosphorylation starts glycolysis. Hexokinase, or glucokinase, catalyze the first committed step.
Q: How is glycolysis regulated?
A: Hexokinase, phosphofructokinase, and pyruvate kinase are the main regulatory enzymes modulated by energy status. ATP inhibits phosphofructokinase when ATP demand is low.
Q: What happens to pyruvate after glycolysis?
A: In aerobic organisms, pyruvate enters mitochondria to fuel the TCA cycle. In anaerobic conditions, organisms convert pyruvate into compounds as lactate or ethanol, through fermentation.
Q: Why is glycolysis studied so extensively?
A: As one of the most ancient metabolic pathways conserved across species, understanding glycolysis provides pivotal insight into core biochemical pathways and their evolution.
