Introduction
The Ectomycorrhiza or ectomycorrhizal root are characterized by the presence of three structural components:
- a sheath or mantle of fungal tissue which encloses the root;
- a labyrinthine inward growth of hyphae between the epidermal and cortical cells called the Hartig net.
- an outwardly growing system of hyphal elements (the extraradical or external mycelium) which form essential connections both with the soil and with the sporocarps of the fungi forming the ectomycorrhizas.
Almost all of the plants upon which ectomycorrhizas develop are woody perennials. Ectomycorrhizal associations are formed predominantly on the fine root tips of the host, which are unevenly distributed throughout the soil profile, being more abundant in topsoil layers containing humus, than in underlying layers of mineral soil (Meyer 1973, Harvey et al. 1976). ECM fungi can make a significant contribution to the biomass of forest ecosystems

Plant Species involved in Ectomycorrhiza
Figure 3. Longitudinal section of fossil Pinus ectomycorrhiza from the middle Eocene Princeton chert of southern British Columbia, Canada. A dichotomous root tip is evident. Figure 4. Transverse section of fossil Pinus ectomycorrhiza from the same material as in Figure 3 showing the excellent preservation of root structure and remnants of the Hartig net (arrowheads). Both were reprinted with permission from LePage et al. Am. J. Bot. 84: 410–412 (1997).
Ectomycorrhizas are usually found on tree species although a few shrub and herbaceous species may also develop this association. Conifer genera such as Picea (spruce), Pinus (pine) and Larix (larch, tamarack) form vast tracts of the boreal forest in the northern hemisphere whereas other conifer species such as Pseudotsuga menziesii (Douglas-fir) and Picea.
Ectomycorrhizal species in angiosperm genera, including Alnus (alder), Betula (birch), Fagus (beech), and Quercus (oak), occur widely in north-temperate forests of the world. In the southern hemisphere, the very large angiosperm genus, Eucalyptus, and several genera in the family Dipterocarpaceae are the dominant ectomycorrhizal tree species.
Fungal species involved in Ectmycorrhiza
The majority of fungal species involved in the ectomycorrhiza symbiosis belong to families in the Basidiomycotina (basidiomycetes), with a few species belonging to the Ascomycotina (ascomycetes). One genus in the Zygomycotina, Endogone, can form ectomycorrhizas (Smith and Read 1997).
Approximately 5,500 known species of fungi are able to form ectomycorrhiza. Of these, about 80% are epigeous, having reproductive structures occurring above ground, and fewer species are hypogeous, producing reproductive structures that remain underground.
Fewer species are hypogeous, producing reproductive structures that remain underground. Hypogeous species, including the truffles, are not as easily detected and more challenging to locate. Both epigeous and hypogeous reproductive structures are often used as indicators of the fungal species present on neighbouring tree roots.
Structures, Developmental Stages and Functions in Ectomycorrhioza
The sequence of events that results in ECM formation has been described in many studies (Chilvers & Gust 1982, Kottke & Oberwinkler 1986, Massicotte et al. 1987, Peterson et al. 2004). These events are summarised below.
1. Root Systems
Most ECM roots have a modified lateral root branching pattern. This pattern, which is called heterophile, consists of short mycorrhizal lateral roots (called short roots) supported by a network of long roots. Short roots normally grow much more slowly than long roots.).
The restricted growth of short roots may be necessary to allow ECM fungi time to form an association since these fungi have difficulty colonizing more rapidly growing roots. The long roots of many species rapidly develop a periderm, which prevents them from forming mycorrhizas.
2. Soil Hyphae
Mycorrhizal fungi produce a hyphal network in soil consisting of individual strands of hyphae and/or relatively undifferentiated bundles of hyphae called mycelial strands, or rhizomorphs with specialized conducting hyphae (Agerer 1995).
Sclerotia, which are larger, resistant storage structures, may also be produced. Soil hyphae function by acquiring nutrients re-allocating resources for fungus reproduction or mycorrhizal exchange and by functioning as propagules for survival and spread of the fungus (Ogawa 1985, Agerer 1995, Unestam & Sun 1995).
3. Root Contact and Hyphal Proliferation: Ectomycorrhiza
It is well known that plant roots release compounds into their immediate environment and the importance of such exudates, mainly as nutrients for the general microbial population, is evidenced by the enhancement of this community in the rhizosphere.
Hyphae contact, recognize and adhere to root epidermal cells near the apex of young, actively growing, high-order, lateral root. These laterals are called short roots because they normally have restricted growth. These show attached hyphae on the root surface 1-2 days after the first contact with the root and the mantle and Hartig net after 2-4 days.
4. Mycorrhizal Roots
After ECM associations are established, mycorrhizal short roots often continue to grow by elongation and branching. Conifer roots with ECM have dichotomous branching patterns, while angiosperms have sympodial branching. The size, colour, texture, and branching patterns of ECM roots vary with different host-fungus combinations, which are called morphotypes.
5. The Hartig Net
Hyphae penetrate between host cells and branches to form a labyrinthine structure called the Hartig net. Hartig net is produced by hyphae that penetrate between the outer cells of the root axis. This penetration is normally from the inner mantle) but can occasionally, for example in the case of Picea abies, occur as soon as hyphae reach the root surface and hence before the mantle is formed.
The depth of this penetration differs in angiosperms and gymnosperms. In most angiosperm species, such as Eucalyptus, Betula, Populus, Fagus, Shorea, etc., the Hartig net develops only around epidermal cells; further, development is blocked by the suberized walls of underlying exodermal cells.
One notable exception is the genus Dryas, where Hartig net hyphae develop around cells in much of the cortex. In the tree species Pisonia grandis, and perhaps a few other species, a Hartig net fails to develop.
The most pronounced feature of the Hartig net is its complex hyphal branching, often referred to as labyrinthine in nature, where tubular fungal hyphae are replaced by a multi-digitate mode of growth (Figure 73).
This increases the surface area for the exchange of nutrients. The cytoplasm of these highly branched hyphae is often enriched with organelles, including mitochondria, which evidence for enhanced metabolic activity. Dolipore septa, typical of basidiomycetes, are frequent in Hartig net hyphae.
Functions Ectomycorrhiza
The Hartig net is involved in nutrient exchange in that it is through these hyphae that most of the sugars are absorbed by the fungus and mineral nutrients and water are passed to the root cells. Autoradiographic analysis has shown the pathway of sugars from root cells into Hartig net hyphae and then into the mantle and phosphate from the mantle into the Hartig net and eventually into the root cells (Bücking and Heyser 2001).
In both conifers and angiosperms, there is a gradient along the length of each mycorrhiza with the most active hyphae being located close to the root tip and senescence of hyphae occurring further back. Hartig net hyphae may also function as a depository for soluble and insoluble carbohydrates, lipids, phenolic compounds, and polyphosphates.
6. Mantle
It is layers of fungal hyphae covering the root surface.
Development
Fungal hyphae contact the surface of lateral roots and may interact with root hairs, the root cap, or the surface of epidermal cells. Interactions between fungal hyphae and root hairs can also be seen in a sectional view. In some cases, root hairs respond by developing thickened cell walls. In most cases, hyphae first contact root cap or epidermal cells.
Once contact is made, the morphology of hyphae changes in that considerable branching occurs as well as an increase in hyphal diameter with continued hyphal growth, the root hairs and old root cap cells are incorporated into the developing mantle and the root surface may be enveloped by loosely organized hyphae or compact hyphae.
In the latter case, deposits of polysaccharides and phenolic materials are often present between hyphae. Some ectomycorrhizas develop cystidia in the outer mantle.
In both loosely organized and compact mantle types, bacteria are usually present on the surface of hyphae and between hyphae. In many ectomycorrhizas, differences occur between the inner, middle, and outer mantle. Inner mantle hyphae frequently branch repeatedly, increasing the surface area for the exchange of nutrients.
Functions Ectomycorrhiza
Although the main nutrient exchange interface in most ectomycorrhizas is the Hartig net, the repeated branching of inner mantle hyphae suggests that these may also be involved in bi-directional movement of nutrients. The fungus is capable of absorbing glucose and /or fructose from root cells and converting these into the soluble carbohydrates, trehalose and mannitol, or into the insoluble carbohydrate, glycogen.
These compounds may either be stored temporarily or for a longer term in mantle hyphae. In addition, mantle hyphae may accumulate other compounds including lipids, proteins, phenolics and polyphosphates.
The compact nature of mantles of some ectomycorrhizas may contribute to the protection of roots from water loss as soils dry and from ingress of pathogenic organisms. Loosely organized mantles probably have little impact on these processes. Since the mantle interfaces with the soil, it potentially affects the transport of water and nutrient ions into the root.
Some bacterial species located on the surface of mantle hyphae (“mycorrhiza helper bacteria”) are known to enhance mycorrhiza formation and consequently plant performance (Garbaye 1994); others are detrimental to mycorrhiza development. Some bacteria associated with the mantle are able to fix atmospheric nitrogen, the fixation products of which can be utilized by the plant.
7. Extra-radical Mycelium Ectomycorrhiza
Hyphae that develop from the outer mantle into the surrounding soil are referred to as extraradical (extra matrical) mycelium.
Development
It is evident that the extraradical mycelium can be an extensive network consisting of mycelial fans that permeate the soil and that may interconnect roots of the same plant and (or) other adjacent plants. The mycelium network often consists of hyphae and rhizomorphs, and in Cenococcum hyphae are thick-walled and pigmented
Some fungi, particularly in the genera Hysterangium and Gautieria, form ‘mats’ of mycelium that bind the soil and fine roots. This mycelium that may be encrusted with calcium oxalate crystals comprises a significant portion of the biomass in forest soils.
Rhizomorphs:
Many ectomycorrhizal fungal species form complexes of extraradical hyphae (rhizomorphs or hyphal strands) that can vary considerably in their morphology, colour, and internal structure.Each rhizomorph (strand) consists of a variable number of individual hyphae that interconnect with each other by different mechanisms.
Some rhizomorphs have calcium oxalate crystal ornamentations of specific size and shape on the surface hyphae and these, along with other features, are often used in species identification.
In the more complex rhizomorphs, one or more central hyphae (vessel hyphae) are enlarged and these may have modified septa that allow for rapid movement of water and nutrient minerals (Cairney 1992). The peripheral hyphae of rhizomorphs may have thickened, pigmented walls that may be involved either in structural support or in providing resistance to the loss of water laterally.
Sclerotia
Relatively few ectomycorrhizal fungal species form sclerotia in the extraradical mycelium. Sclerotia are initiated in the extraradical mycelium and are sometimes associated with rhizomorphs. Hyphae branches, come into contact with each other and form discrete structures.
At maturity, each sclerotium usually develops a melanized outer covering, the rind that surrounds a cortex (central area) of compact hyphae, and a medulla of loosely organized hyphae. Sclerotia are initiated in the extraradical mycelium and are sometimes associated with rhizomorphs.
Hyphae branches, come into contact with each other and form discrete structures. At maturity, each sclerotium usually develops a melanized outer covering, the rind that surrounds a cortex (central area) of compact hyphae, and a medulla of loosely organized hyphae.
Hyphae developing from germinated sclerotia are effective in forming typical ectomycorrhizas. Sclerotia as well as cultured mycelium can be used for the long-term maintenance of fungal genotypes.
Sexual reproductive structures in Ectomycorrhiza
The formation of reproductive bodies, both basidiocarps and ascocarps, involves the localized branching of extraradical hyphae. At maturity, a productive structure typical of the fungal species is formed. Basidiospores in the basidiomycetes, and ascospores in the ascomycetes formed in the reproductive structures, are dispersed by various mechanisms as propagules for the fungus.
Functions
The extraradical mycelium, highly variable in structure, has a significant role in ectomycorrhiza functioning. The most obvious function of the fine hyphae that comprise much of the extraradical mycelium is the mobilization, absorption and translocation of mineral nutrients and water from the soil substrate to plant roots.
In those species with rhizomorphs, connecting fine hyphae may pass water and dissolved nutrients to these structures for more rapid translocation through the wide-diameter central hyphae (vessel hyphae) to the root. At some point, the extraradical hyphae and rhizomorphs interface with the mantle where transfer must occur to outer mantle hyphae.
The ectomycorrhizal fungi can obtain phosphorus from the mycelium network of a saprotrophic fungus and pass phosphorus to the host plant. Carbon compounds are translocated in the reverse direction from the host root to the extraradical mycelium for metabolic and growth processes, to developing sclerotia and their storage reserves, and to sexual reproductive bodies (e.g., basidiocarps and ascocarps) that act as major sinks for host-derived carbon.
8. Specialized ectomycorrhizas – tuberculate mycorrhizas:
When we associated with certain fungal species, several conifers, including some Pinus species (Randall and Grand 1986), Pseudotsuga (Massicotte et al. 1992), and Tsuga as well as several angiosperms such as Eucalyptus (Dell et al. 1990), Quercus, Castanopsis and others, develop clusters of lateral roots known as tubercles.
Each lateral root in the cluster develops a mantle and Hartig net, while the entire cluster of roots becomes covered, to a variable degree by compact layers of hyphae called the peridium or rind.
Hartig net hyphae and inner mantle hyphae may store polysaccharides and proteins. The suggestion has been made that the rind may protect the enclosed ectomycorrhizas from pathogens and insects.
Bacteria that have been isolated from the surface and within the peridium of Pseudotsuga tubercles are able to fix atmospheric nitrogen; it is not known whether this nitrogen is utilized by the host.
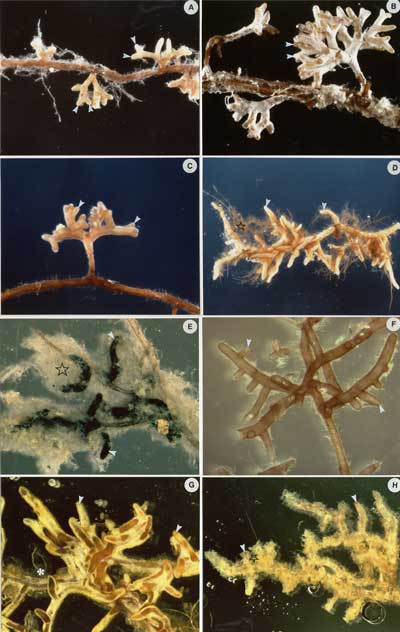
Source: Figure 4.7 in Brundrett et al. 1996
References:
- Mycorrhizae Book By Smith 1997
- Mycorrhizael Anatomy and molecular biology Book by Pate
- Mycorrhizae.info

Usually I do not read post on blogs, however I would like to say that your blog is very pressured to read! Your writing style has been amazed me. Thanks, very nice post.
Awesome post, thanks for the useful information.
It is appropriate time to make a few plans for the long run and it is time to be happy. I’ve learn this post and if I may I wish to recommend you few fascinating things or suggestions. Maybe you can write subsequent articles relating to this article. I desire to learn more things about it!
These are in fact wonderful ideas in about blogging. You have touched some good points here. Any way keep up wrinting.
*You should take part in a contest for one of the best blogs on the web. I will recommend this site!
I抎 need to verify with you here. Which isn’t something I often do! I enjoy studying a submit that will make individuals think. Also, thanks for allowing me to comment!
Thank you for some other great article. The place else may just anybody get that kind of info in such a perfect method of writing? I’ve a presentation subsequent week, and I’m on the search for such information.
Like!! I blog frequently and I really thank you for your content. The article has truly peaked my interest.
Do you mind if I quote a couple of your posts as long
as I provide credit and sources back to your website? My website is
in the very same niche as yours and my visitors would definitely benefit from a lot of the information you provide here.
Please let me know if this okay with you. Thanks a lot!
Thanks a lot for the post.Really thank you! Much obliged.buy viagra prof