Pure air is colorless and odorless. But various pollutants from natural and artificial sources are entering the atmosphere daily, disturbing the dynamic equilibrium. This leads to air pollution when the normal properties of air are upset, and both man and the environment suffer.
Natural sources of air pollution are:
• Volcanic activity, vegetation decay, forest fires emitting carbon monoxide, sulphur dioxide and hydrogen sulphide and tiny particles of solids or liquids sprayed from the seas and land by the wind.
Man-made sources are:
• Gases, mists, particulates and aerosols emitted by industries and other chemical and biological processes used by man.
Primary Pollutants
Five primary pollutants together contribute more than 90 percent of global air pollution:
- Carbon monoxide, C.O.
- Nitrogen oxides, NOX
- Hydrocarbons, H.C.
- Sulfur oxides, SOX and
- Particulates.
Transportation accounts for more than 46 percent of the total pollutants produced annually and remains the principal source of air pollution. Carbon monoxide is the major industrial pollutant, with a tonnage matching that of all other contaminants. However, particulate pollutants, though minor, are the most dangerous among the primary pollutants (100 times more harmful than carbon monoxide).
Table: Primary Air Pollutant sources and Their Quantities (Million Tons per Year).
The above data are taken from those in the USA (1990). The USA and other developed countries contribute most to air pollution.
Carbon Monoxide, CO
It is a colorless, odorless, tasteless gas that is dangerous to our health. Each year 350 million tons of CO (275 million tons from human sources and 75 million tons from natural sources) are emitted worldwide in, which the USA alone shares 100 million tons.
Transportation accounts for 70 percent of C.O. emissions. That is to say, diesel and petroleum engines in automobiles are primarily responsible for about 70 percent of C.O. emissions. The sources of carbon monoxide, C.O. are the chemical reactions:
(i) incomplete combustion of fuel or carbon-containing compounds:
2C + O2 ————–> 2CO
(carbon) (oxygen) (carbon monoxide)
(ii) reaction of carbon dioxide and carbon-containing materials at elevated temperatures in industries, e.g., in blast furnaces:
CO2 + C ———————————> 2CO
(carbon dioxide) (carbon)
(iii) dissociation of carbon dioxide at high temperatures:
CO2 —————- C.O. + O
Sinks
Part of Carbon monoxide is lost in the upper atmosphere. The major sink is soil micro-organisms. A potting soil sample weighing 28 kg can completely remove in 3 hours 120 ppm carbon monoxide from ambient air. The same soil sample on sterilization failed to remove carbon monoxide from the air.
Control of C.O. Pollution
Petroleum and diesel-fed automobiles account for the major share of carbon monoxide emissions. Hence efforts for carbon monoxide pollution control are mainly aimed at automobiles. The use of catalytic converters in automobiles’ internal combustion engines helps clean up the exhaust emissions.
Such converters built into the automobile engines promote oxidation-reduction cycles and ensure complete combustion of carbon monoxide, nitrogen oxides and hydrocarbons. The following figure and flow sheet illustrate the action of catalytic converters: The use of catalytic converters in two stages helps in
elimination of pollutants from exhaust gases before they are discharged into the atmosphere.
In the first converter, nitrogen oxides are reduced to nitrogen (+ ammonia) in the presence of finely divided catalyst platinum and the reducing gases, carbon monoxide and hydrocarbons. The production of ammonia is kept at a minimum under carefully controlled conditions.
In the second converter, the air is introduced to provide an oxidizing atmosphere for the complete oxidation of carbon monoxide and hydrocarbon into carbon dioxide and water in the presence of a finely divided platinum catalyst.
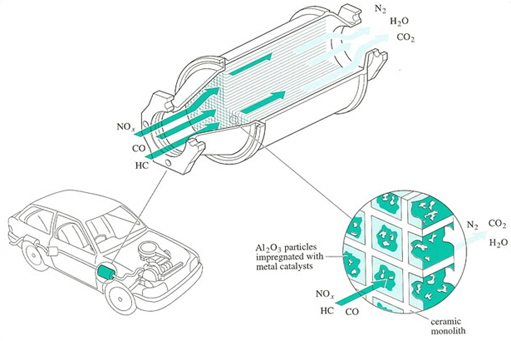
Thus by means of platinum catalytic converters, auto exhaust emissions are cleaned up through reduction-oxidation reactions. In all developed countries, it is mandatory by law for all automobiles to fit their engines with catalytic converters. Some automobile companies in India plan to fit their automobile engines with catalytic converters.
Nitrogen Oxides, NOX
It consists of mixed oxides, nitric oxide and nitrogen dioxide (NO and NO2, respectively)—the former is a colorless and odorless gas, but the latter (NO2) has a reddish-brown color and pungent smell.
The formation of NO and NO2 is based on the chemical reactions:
These reactions occur inside the automobile engines, so the exhaust gases consist of NOX. The latter concentration in rural air is much less than in urban air. In the air, NOX is converted into nitric acid, HNO3 by natural processes:
This nitric acid is one of the constituents of acid rain discussed in a subsequent section. From auto exhaust emissions, NOX is removed, as discussed above, through catalytic converters.
Hydrocarbons and Photochemical Smog
Natural processes, particularly trees, emit large quantities of hydrocarbons into the air. Methane, CH4, is a major hydrocarbon. It is generated in large amounts by bacteria formed by the anaerobic decomposition of organic matter in water, sediments and soil.
Domestic animals (cattle, buffaloes, etc.) contribute about 85 million methane annually to the atmosphere. Auto-mobiles are significant sources of hydrocarbons. In the presence of ozone, carbon monoxide, nitrogen oxides and hydrocarbon participate in photochemical reactions (in the presence of sunlight). A chain reaction proceeds in which the free radical R CH2• is generated in the first step.
Other free radicals which are formed are: R CH2O2• in the second step by reaction with oxygen, R CH2O•; R CH2O• in the third step by reaction with nitric oxide; HO2• in the fourth step by reaction with oxygen—a stable aldehyde R CHO is another product at this stage; HO• is formed in the fifth step by reaction with nitric oxide (nitrogen dioxide is another product here); and finally, the starting free radical R CH2• is regenerated by reaction with hydrocarbon, R CH3 thereby sustaining the chain reaction.
The harmful products in the chain reaction are NO2 and aldehyde, R CHO. A side reaction is followed by another route through the aldehyde, R CHO; it gives a dangerous end product, peroxy acyl nitrate (PAN), a strong eye irritant.
These reactions lead to photochemical smog formation, characterized by brown, gray fumes that irritate the eyes and lungs and cause serious plant damage. Photochemical smog occurs in coastal cities in a winter climate, e.g., in Los Angeles, the USA, which has the heaviest vehicular traffic.
Sulphur Dioxide, SO2
Sulfur dioxide is a colorless gas with a pungent odor. It is produced from the combustion of any sulfur-bearing material. Sulfur dioxide, SO2, is always associated with a little sulfur trioxide, SO3.
Man-made sources—coal-fired power stations and other industries contribute about 33 percent of SOX pollution, while natural sources, viz. volcanoes, provide about 67 percent of SOX pollution. Soot particles containing metal oxides catalyze the oxidation of sulfur dioxide to trioxide.
The first reaction above occurs in the presence of ozone and water vapor. The product, sulphuric Acid, is formed on aerosol (fine particles suspended in the air, smoke, fog, mist, etc.) droplets. Sulphuric Acid is one of the constituents of acid rain. In winter, sulfur oxides from thermal power plants, along
with other gases, lead to smog formation, e.g., London smog.
This is known as reducing smog in contrast with photochemical smog, known as oxidizing smog (consisting of hydrocarbons, nitrogen oxides and ozone). London smog (1952) is well-known for its disastrous effect. Heavy pollution (SO2) conditions prevailed in London for five days, killing about
4,000 people. The causes of death were bronchitis, pneumonia, and other respiratory troubles, particularly among aged people.
Control of SOX Pollution
SOX (sulfur oxides) from flue gases of industrial plants can be removed using chemical scrubbers. The flue stack gases are led through a bed of (slurry) of limestone, CaCO3 (calcium carbonate), which absorbs sulfur dioxide quite efficiently. The method is economical, but the disposal of solid waste,
calcium sulfate is a problem.
Alternatively, sulfur oxide in an aqueous solution is treated with citric acid salt, and the resulting solution is exposed to a stream of hydrogen sulfide gas whereby sulfur is deposited. This sulfur can then be recovered and utilized.
Thermal power plants, major sources of man-made SOX pollution, are normally constructed with tall chimneys to disperse the emissions over a wide area. This reduces the local problem but creates problems for faraway places through acid rains (see below).
Acid Rain
As described above, many nitrogen oxides, NOX and sulfur oxides, and SOX entering the atmosphere are transformed into Nitric Acid (HNO3) and sulphuric acid (H2SO4) respectively. These combine with hydrogen chloride, HCl from HCl emissions (both by man-made and natural sources) and
generate acidic precipitation, known as acid rain.
Acid rain is a major environmental issue as it badly damages the environment. It damages buildings and structural materials of marble, limestones, slate and mortar. These materials become structurally weak as calcium carbonate reacts with sulphuric acid to form soluble sulfate, which is leached out by rainwater:
Fig. 6.6 Acid rain in Greece and Italy
In Greece and Italy, invaluable stones and statues have been partly dissolved by acid rain. Besides these, acid rain damaged forests in Germany and lakes in Sweden and Canada. Acid rain originated in the U.K. but far away in Sweden; it hurt some 8,000 lakes, of which 4,000 are dead. Taj mahal stones are affected by acid rain.
Control of Acid Rain
As discussed above, acid rain can be checked if its constituents, sulfur oxide and nitrogen oxide, are controlled.
Particulate
Small solid particles and liquid droplets are collectively termed particulates. They originate both from natural and man-made sources. Natural sources discharge 800–2,000 million tons annually, and man-made sources 200–500 million tons of particulates. Among man-made sources, fly ash from thermal power plants deserves mention. Table 6.3 lists the annual production of particulate matter from the two sources.
Table 2. Worldwide Addition of Particulate Matter to the Atmosphere (in Million Tons)
Particulates range in size from 0.0002 µ (about the size of a molecule) to 500 µ (l µ = 10–6 meters). The number of particles in the atmosphere varies from several hundred per cm3 in clean air to more than 100,000 per cm3 in highly polluted air (urban/ industrial areas).
Soot
Soot particles originate from fuel combustion and comprise the highly condensed product of polycyclic aromatic hydrocarbon (PAH)—roughly 100 condensed aromatic rings. The hydrogen content of soot is 1–3 percent, and oxygen content is 5–10 percent due to partial surface oxidation.
Due to the large surface area, soot acts as a carrier for toxic organics, e.g., benzo-α -pyrene and toxic trace metals, e.g., beryllium, cadmium, chromium, manganese, nickel, vanadium, etc. A soot particle has an average size of 0.1–20 µ. The finer particles (< 3 µ ) are the worst causes of lung damage due to their ability to penetrate deep into our respiratory tract and thence into the lungs, where they remain for years and cause all sorts of diseases such as cough, bronchitis, asthma, and finally cancer.
Particulates cause increased corrosion of metals which assume serious dimensions in industrial and urban areas. They are responsible for damage to buildings, sculptures, paintings, etc.
Fig. 3. A soot particle
Particulates play key roles in the atmosphere. They reduce visibility by scattering and absorption of solar radiation. They influence the climate by forming clouds, rain and snow by acting as nuclei upon which water can condense into raindrops. Atmospheric particulate levels can be correlated with the extent of precipitation over cities and suburbs.
Control of Particulate Emissions
The removal of particulate matter from gas streams is an essential step for air pollution control. The best equipment is the Electrostatic Precipitator. An electrostatic precipitator is based on the principle that aero-sol particles acquire a charge when subjected to an electrostatic field.
Air Pollution and Biosphere
Air pollutants are present largely in the troposphere and lower stratosphere. The ground air, l–100 meters high, is very much polluted in urban and industrial areas. Some pollutants are absorbed on vegetation, buildings and water surfaces. The primary pollutants discharged into the atmosphere undergo chemical changes in the presence of water vapor, oxygen and solar ultra-violet radiation and produce secondary pollutants.
These pollutants (secondary) have harmful effects on soil, vegetation, crops, animals, men and materials.
Plants are affected both by gaseous pollutants and by particulates deposited on soil. Acid rain over some time tends to reduce the soil pH (= log H+, i.e., the negative logarithm of hydrogen ion concentration which is an index of acidity, alkalinity or neutrality) and renders it acidic and less fertile.
Moreover, the deposition of toxic metals on soil in industrial areas makes the soil unsuitable for the growth of plants. Some plants are very sensitive to traces of toxic metals as the latter inhibit the action of some plant enzymes. Particulates such as dust and soot are deposited on plant leaves and block the stomata (opening in the epidermis of plants).
This restricts the absorption of carbon dioxide and reduces the photosynthesis and transpiration rate. The overall result is retarded growth of plants and decreased yield of crops. In California, the USA, sulfur dioxide in the air and metallic pollutants in soil killed vegetation in an area of 300 km2 and affected growth on a further 350 km2 land.
In Leeds, U.K., there was a drastic decrease in the growth of lettuce and radish in heavily polluted industrial areas compared to less polluted areas of the city. Ozone and peroxy acyl nitrate (PAN) (in photochemical
smog, see the previous section) are oxidizing agents which attack plants by oxidizing their sulphydryl (–S.H.) groups of proteins into disulfides.
This leads to inhibition of individual enzyme activity. They also affect photosynthesis by plants. Cattle are affected by air pollution, particularly under smog conditions. They develop breathing troubles and loss of appetite and show low milk yield while many of them die. Man has become the victim of air pollution. Thousands of chemicals pose problems of health hazards during manufacture and handling.
The United has submitted a typical list of 24 extremely hazardous substances in the atmosphere.
States Environmental Protection Agency (1973):
- Acrylonitrile, Arsenic, Asbestos, Benzene, Beryllium, Cadmium, Chlorinated solvents, Chlorofluorocarbons, Chromate, Coke oven emissions, Ethylene oxide, Lead, Mercury, Ozone, Sulphur dioxide, Vinyl chloride, Toxic waste disposal emissions and leachates (washings), etc.
Meteorology and Air Pollution
Air pollution, one of the man-made activities, impacts meteorology, i.e., the science of atmospheric phenomena. Meteorology is based on physical parameters such as temperature, wind, moisture, and movement of air masses in the atmosphere. It is also affected by the chemical properties
of the atmosphere and the chemical reactions going on in the atmosphere.
The air pollutants get dispersed in the atmosphere depending on the air circulation patterns. In this context, temperature inversion plays an important role. It occurs when a warm air mass moves above a cold air mass resulting in air stagnation of the latter (cold air) in which air pollutants get trapped.
The air above the ground becomes polluted. This happens when warm air blows over a mountain range and cool air on the other side of the field. This phenomenon is observed in Denver, USA, east of the Rocky Mountains.
Human activities are partly responsible for changing the meteorology of the earth.
References
- ChemIDplus – 7440-59-7 – National Institutes of Health. https://chem.nlm.nih.gov/chemidplus/rn/7440-59-7
- 88 Environment and Ecology – Our Education. http://upsc.oureducation.in/wp-content/uploads/2016/11/Environment-Ecology-101-193.pdf
- Sulfur Dioxide | Public Health Statement | ATSDR. https://wwwn.cdc.gov/TSP/PHS/PHS.aspx?phsid=251&toxid=46
- ENVIRONMENTAL – جامعة تكريت. https://ceng.tu.edu.iq/ched/images/lectures/chem-lec/st2/c4/lecture%20nots%20environmantal%20engineering/lect.5.pdf
- Environmental Education – PDF Free Download – Donuts. https://epdf.pub/environmental-education.html
- JP3358008B2 – Electric dust collector – Google Patents. https://patents.google.com/patent/JP3358008B2/en

Hello There. I found your blog using msn. This is a very neatly written article. I抣l make sure to bookmark it and return to read more of your helpful information. Thanks for the post. I抣l definitely return.
If some one wants expert view on the topic of blogging and site-building after that i advise him/her to visit this website, Keep up the fastidious job.
Keep up the good work, thanks!
I have been absent for some time, but now I remember why I used to love this site. Thank you, I will try and check back more frequently. How frequently you update your site?
thnaks
It is perfect time to make a few plans for the long run and it’s time to be happy. I have read this submit and if I may I want to counsel you some fascinating issues or suggestions. Perhaps you can write next articles regarding this article. I desire to learn more issues approximately it!
Wow, amazing blog layout! How long have you been blogging for? you make blogging look easy. The overall look of your website is fantastic, as well as the content!
Thanks sir
I was very pleased to search out this net-site.I wanted to thanks for your time for this glorious learn!! I positively enjoying every little bit of it and I have you bookmarked to check out new stuff you blog post.
I happen to be writing to let you understand what a fine encounter our princess found studying your webblog. She discovered too many details, including how it is like to possess an awesome teaching spirit to have many more completely learn specific tortuous subject areas. You truly exceeded her expectations. I appreciate you for giving these effective, safe, revealing not to mention unique tips on the topic to Kate.
A lot of thanks for all of the effort on this web page. Kate really loves working on investigation and it’s obvious why. A lot of people know all regarding the compelling mode you present good tips and tricks by means of your web blog and therefore improve response from the others on that situation so our favorite princess is certainly learning a lot. Have fun with the rest of the year. You’re the one doing a powerful job.
I needed to compose you this bit of word so as to thank you very much once again for these striking information you’ve shared in this case. This is quite seriously generous with you giving unreservedly what exactly some people could have sold for an e-book to help with making some bucks for themselves, specifically since you might well have done it in the event you considered necessary. Those concepts as well worked to become great way to fully grasp that other people have the identical dream like mine to grasp a little more around this issue. I’m sure there are millions of more enjoyable periods up front for many who looked over your site.
I am also writing to let you be aware of what a really good discovery my cousin’s girl developed visiting your webblog. She learned several issues, which included how it is like to possess an awesome giving heart to make other people quite simply know just exactly a variety of advanced subject matter. You really surpassed her desires. Thank you for displaying these informative, trusted, educational and in addition cool thoughts on your topic to Sandra.
Thank you for every one of your effort on this website. My mother loves working on research and it’s really simple to grasp why. Almost all notice all concerning the dynamic medium you deliver invaluable techniques via the website and as well inspire participation from people on that article so our girl is actually being taught a great deal. Take advantage of the remaining portion of the year. Your performing a good job.
I simply wanted to construct a quick word so as to say thanks to you for those fantastic instructions you are giving at this site. My prolonged internet research has at the end been compensated with excellent facts to go over with my great friends. I ‘d say that most of us readers actually are rather endowed to live in a useful website with many marvellous people with interesting methods. I feel extremely lucky to have seen your weblog and look forward to plenty of more pleasurable times reading here. Thanks a lot once more for all the details.
I and also my friends have been examining the best suggestions found on your web page while the sudden came up with a terrible feeling I had not expressed respect to the site owner for those tips. Most of the young boys ended up for that reason very interested to learn them and already have in fact been having fun with these things. Thank you for being well accommodating as well as for utilizing such wonderful subject matter millions of individuals are really wanting to discover. My very own honest apologies for not expressing gratitude to you earlier.
I as well as my pals were actually reading through the nice solutions on the blog and so the sudden developed a terrible feeling I had not expressed respect to the website owner for those strategies. Those boys ended up as a consequence stimulated to read them and have in reality been enjoying them. We appreciate you being very helpful and then for deciding on certain marvelous guides millions of individuals are really eager to be informed on. My very own sincere regret for not expressing appreciation to sooner.
A lot of thanks for all your valuable labor on this website. My mum takes pleasure in carrying out investigation and it is simple to grasp why. My partner and i know all relating to the lively medium you give simple guidance via the blog and cause participation from website visitors about this content plus our own simple princess is always studying a great deal. Enjoy the rest of the year. You are always doing a great job.
I and also my pals were digesting the excellent helpful tips found on your web blog and so all of a sudden developed an awful suspicion I had not expressed respect to the web site owner for those techniques. My young boys were definitely consequently very interested to study them and already have extremely been tapping into them. Appreciate your indeed being quite thoughtful as well as for pick out such ideal information most people are really desirous to learn about. Our own honest apologies for not expressing appreciation to you sooner.
Thank you so much for giving everyone such a wonderful opportunity to read from this web site. It is always very pleasant and stuffed with fun for me and my office fellow workers to visit your website minimum thrice every week to read the fresh guides you have. And lastly, I’m actually astounded with all the staggering concepts you give. Selected two tips in this posting are unquestionably the best I’ve ever had.
I and my friends were examining the good procedures located on your website and so suddenly I had a terrible feeling I had not thanked the blog owner for them. These men had been for this reason thrilled to read all of them and have pretty much been loving these things. I appreciate you for truly being well helpful as well as for choosing this form of high-quality tips millions of individuals are really desirous to be informed on. My sincere apologies for not saying thanks to you sooner.
Thanks a lot for giving everyone a very pleasant possiblity to read critical reviews from this web site. It really is so kind plus packed with a lot of fun for me and my office fellow workers to search the blog the equivalent of three times in 7 days to study the new tips you will have. Not to mention, I am usually amazed with the stunning creative ideas served by you. Certain two areas in this posting are unquestionably the most suitable I’ve had.
I want to show my affection for your kind-heartedness supporting visitors who absolutely need guidance on this important situation. Your personal commitment to passing the message along became astonishingly effective and has frequently enabled guys much like me to get to their targets. This useful information implies a whole lot a person like me and somewhat more to my office colleagues. Best wishes; from everyone of us.