Benedict’s test detects reducing sugars (sugars having a free reactive carbonyl group). It is a qualitative chemical test that detects reducing sugars in a given sample.
Reducing sugars possess a free aldehyde or ketone functional group and can reduce other substances.
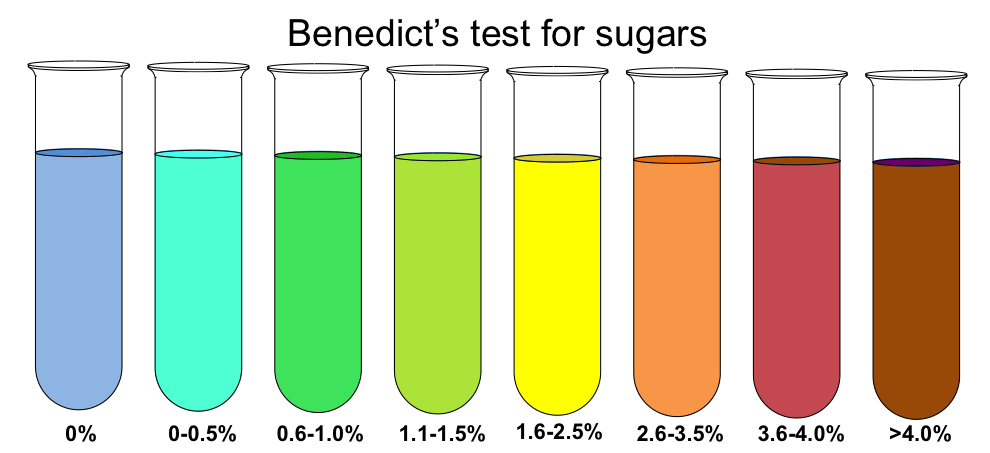
Benedict’s test relies on the ability of reducing sugars to reduce cupric ions (Cu²⁺) present in Benedict’s solution, resulting in a color change from blue to green, yellow, orange, or even brick-red, depending on the amount of reducing sugar present.
- Principle
- Reagents Required
- Procedure
- Result
- Applications of Benedict’s Test
- Limitations of Benedict’s Test
- Safety Precautions
- FAQs
- Q: Can Benedict’s test detect all types of sugars?
- Q: Is it necessary to heat the test tube during the test?
- Q: What is the advantage of using a colorimeter for result quantification?
- Q: Can Benedict’s test be used for quantitative analysis?
- Q: Are there any specific disposal guidelines for Benedict’s test residues?
Principle
This test utilizes a mixture of sodium citrate, copper (II) sulfate, and sodium carbonate in a slightly basic solution. Reducing sugars reduce the copper (II) ions to copper (I) oxide (Red ppt).
R-CHO + 2Cu2+ +5OH- ————————-R-CO2-+Cu2O (red ppt) + 3H2O
R-CHO =Reducing carbohydrates
R-CO2- = Carbohydrate ion
Reagents Required
i. Benedict’s Reagent: Dissolve Sodium Citrate (173 g) and anhydrous Sodium Carbonate (100 g) in about 700 ml of distilled water by gently heating the contents.
Dissolve Copper Sulfate (17.3 g) in a separate beaker in about 100 mL of distilled water. Transfer this solution gradually into the Carbonate-Citrate mixture with constant stirring and raise the volume to 1 L with distilled water.
ii. Carbohydrates (glucose, starch/sucrose)
Procedure
- Add 1 ml of the sample to a test tube.
- Then add 2 ml of Benedict’s reagent.
- Mix the contents thoroughly.
- Heat the solution in a boiling water bath for 3 minutes.
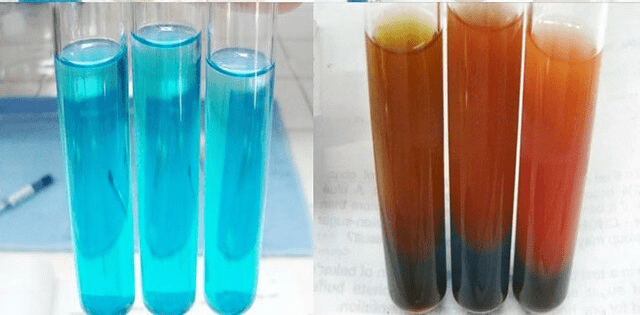
- The formation of a red precipitate indicates a positive test.
Result
The brick red Colour shows a positive test and indicates the presence of reducing sugar.
Applications of Benedict’s Test
Benedict’s test finds application in various fields:
- Food industry: It helps determine the sugar content in food and beverages, such as fruit juices and soft drinks.
- Clinical diagnostics: Benedict’s test assists in diagnosing conditions like diabetes mellitus by detecting the presence of glucose in urine.
- Biochemistry and research: It assesses reducing sugars in biological samples and analyzes enzymatic reactions.
Limitations of Benedict’s Test
Although Benedict’s test is a useful tool, it has certain limitations:
- Specificity: It may give false-positive results with certain non-reducing substances.
- Sensitivity: Benedict’s test is less sensitive than other quantitative methods, such as enzymatic assays.
- Interference: Certain compounds, such as ascorbic acid and some medications, can interfere with the test results.
Safety Precautions
While performing Benedict’s test, it is crucial to follow safety precautions:
- Wear appropriate personal protective equipment, including gloves and safety goggles.
- Handle chemicals with care and avoid contact with skin or eyes.
- Use a fume hood or ensure proper ventilation when heating samples.
- Dispose of chemicals and test residues according to the appropriate guidelines.
FAQs
Q: Can Benedict’s test detect all types of sugars?
Ans: Benedict’s test specifically detects reducing sugars but may not detect non-reducing sugars.
Q: Is it necessary to heat the test tube during the test?
Ans: Yes, heating the test tube is essential as it aids in reducing cupric ions and color change.
Q: What is the advantage of using a colorimeter for result quantification?
Ans: Using a colorimeter provides a more accurate and precise measurement of the color change, enabling quantitative analysis.
Q: Can Benedict’s test be used for quantitative analysis?
A: Benedict’s test is primarily qualitative but can be semi-quantitative if the color change is compared to a standard color chart.
Q: Are there any specific disposal guidelines for Benedict’s test residues?
Ans: Following local regulations and guidelines for properly disposing of chemicals and test residues is essential.
